Mass Cytometry (CyTOF) combines the advantages of time-of-flight mass spectrometry and flow cytometry. It utilizes antibodies labeled with metal isotopes to mark cells (both surface and internal protein molecules). By analyzing the composition of labels on individual cells through time-of-flight mass spectrometry, CyTOF enables detailed and in-depth research into cellular phenotypes and functions. Currently, it can achieve simultaneous detection of over 40 markers at the single-cell level. Since CyTOF employs heavy metal isotope-conjugated antibodies to label cells, rather than fluorescent dyes, it fundamentally breaks through the limitations of traditional fluorescent flow cytometry in terms of channel number.
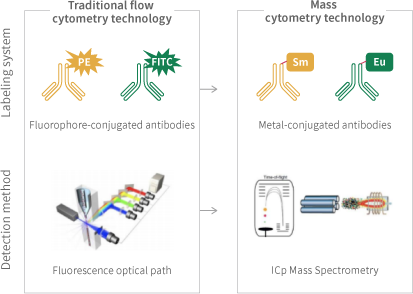
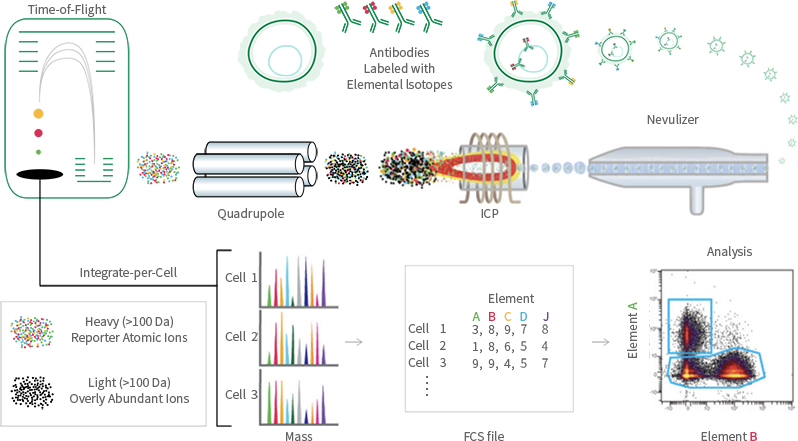
Using heavy metal isotope-conjugated antibodies, protein molecules on the surface and within cells are labeled with metal isotope-tagged antibodies. The labeled cells are then separated into individual cells through a nebulizer. These individual cells enter an inductively coupled plasma (ICP) torch for ionization. The resulting ion clouds formed by the single cells are then directed into a time-of-flight mass spectrometer, where the types and quantities of metal tags carried by each cell are detected. This ultimately yields mass spectrometry data for single cells.
Due to the different mass-to-charge ratios of different metal ions, they have different time-of-flight in the detector (quadrupole). Heavier ions require longer time to traverse the detector.

Full-spectrum flow cytometry is a rapid, sensitive, and high-resolution cell analysis technology developed based on flow cytometry in combination with optical technology. It enables simultaneous multi-parameter detection of individual cells, as well as quantitative and phenotypic analysis of antigens on the cell surface and/or within the cell. When individual cells flow through one or multiple lasers in a full-spectrum flow cytometer, the scattered light information and multiple fluorescent signals from each cell are detected by the detectors, enabling rapid and precise detection, analysis, and characterization of millions of cells in a single sample simultaneously. Currently, it is possible to easily perform immunophenotyping experiments with over 40 colors.
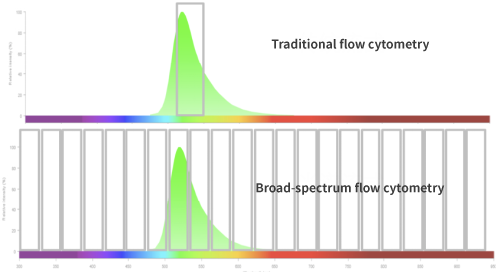
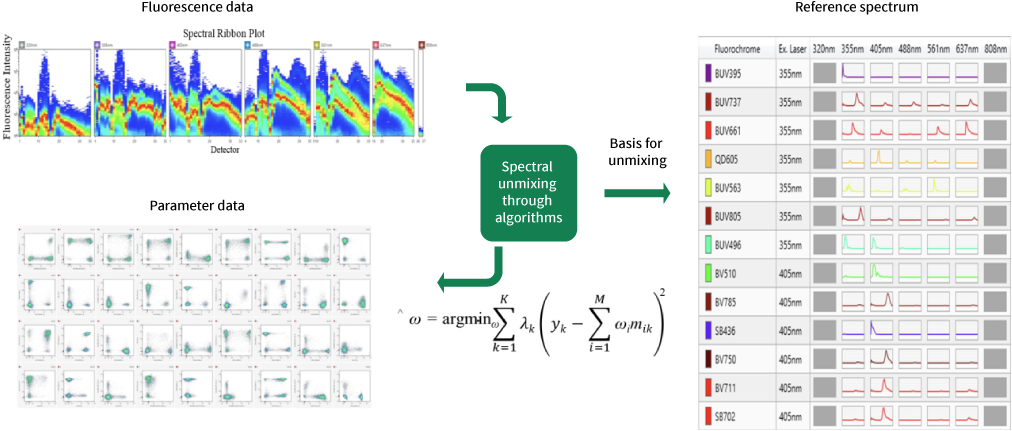
In full-spectrum flow cytometry, multiple detection channels are used to obtain the complete emission spectrum information of fluorescent dyes under each laser. This allows the complete fluorescence spectrum of each fluorescent dye to be identified and recorded for its spectral characteristics, as each fluorescent dye's full spectrum is unique. The complete spectral signal of each cell is a superposition of multiple dye signals. Spectral unmixing is performed through mathematical algorithms, which separate the superimposed full spectrum into individual signal values for each dye based on their unique spectral characteristics. This enables conventional analysis through two-parameter images.
Common applications of full-spectrum flow cytometry include immunology, infectious diseases, tumor immunology, microbiology, biomarker identification, drug discovery, and molecular biology. There is a growing interest in understanding the complexities of the immune system and leveraging immune cells to improve health, which has led to the increasing popularity of high-dimensional cell detection.